Global research into new HIV prevention products stands at a crucial crossroads, and the future of topical microbicides may hang in the balance.
For a quarter century—and in earnest over the past decade—scientists have sought to develop HIV-preventing, antiretroviral (ARV)-infused products known as microbicides that would be inserted into the vagina in the form of sponges, gels, creams, rings or suppositories or into the rectum through enemas, gels, creams or lubricants. Microbicide advocates argue that such products would help address the crucial unmet needs of at-risk individuals who may not prefer or be able to access existing prevention products, including condoms and Truvada (tenofovir disoproxil fumarate/emtricitabine) as pre-exposure prophylaxis (PrEP). Importantly, vaginal microbicides could empower women whose partners refuse to use condoms, arming them with their own discreet form of HIV prevention.
The microbicides field has experienced notable setbacks over the years, but it boasts one key recent success, albeit a relatively modest one: a monthly vaginal ring containing the ARV dapivirine that apparently cuts HIV risk by about 60 percent among women who use it the most consistently.
The dapivirine ring’s progress toward regulatory approval in various nations notwithstanding, a top player at the National Institutes of Health (NIH)—Carl W. Dieffenbach, PhD, the director of the Division of AIDS (DAIDS)—believes that the various types of microbicides currently in earlier stages of development may not prove promising enough to justify the highly expensive large-scale trials necessary to bring any one of them to market.

Carl Diffenbach, PhDCourtesy of NIAD/NIH
Dieffenbach’s stance has ignited a debate within the HIV research and advocacy community over whether ultimately scaling back research and funding for microbicides while expanding support for other forms of prevention, such as vaccines or new types of PrEP, would represent a shrewd strategy or whether such a shift would jeopardize crucial advances in HIV prevention science in favor of uncertain promises.
“I am focused on getting a next generation of products that are safe and desirable and will be used by the most vulnerable at-risk people, young women and men internationally and domestically,” Dieffenbach says. “These products need to fit within the context of these lives.”
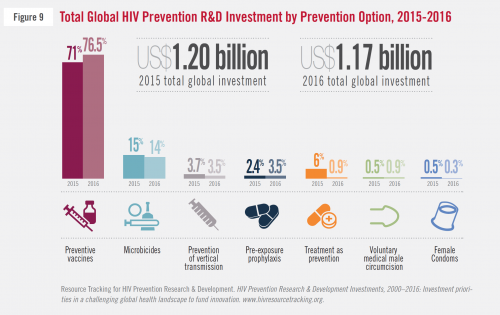
The 2015 and 2016 breakdowns of the proportions of global spending by all funders on HIV prevention research and investment, according to type of prevention.AVAC
The public debate has been spurred by the fact that DAIDS, a division of the NIH’s National Institute for Allergy and Infectious Diseases (NIAID) that funds five major HIV clinical trial networks, is undergoing a standard seven-year review of its scientific priorities. DAIDS’s goal is to communicate to the HIV research community by mid-2018 details about the types of grants the agency seeks to award for the 2020 to 2027 funding cycle.
This is a moment of reckoning for researchers working in HIV-prevention research and development. Overall, the U.S. federal government contributed three quarters of the $1.17 billion spent worldwide in the field in 2016, including 84 percent of the $167 million that went to microbicides research. Such financial dominance affords the NIH immense power to steer major global research endeavors.

The United States is by far the dominant force behind global spending on HIV prevention research and development.AVAC
A Controversial Vision
Dieffenbach invited the public to comment on DAIDS’s funding priorities during a period that closed at the end of November. At the same time, he made it clear that he believed future U.S. investment should prioritize HIV prevention methods that are 1) long-acting (working for six months or longer) and thus less dependent on adherence; and 2) systemic, meaning they provide protection to the entire body and not just the rectum or vagina.
In another controversial position, Dieffenbach has also proposed that all scientists seeking grants for non-vaccine-related HIV prevention compete for the same pool of funding. In the past, the applications for such research grants has been siloed through two DAIDS-funded clinical trials networks that focus on prevention—the HIV Prevention Trials Network (HPTN) and the Microbicides Trial Network (MTN).
Dieffenbach, who says that “getting a safe, effective and durable HIV vaccine is [the NIH’s] highest priority,” expresses particular concern that the vaginal ring’s efficacy among the African women who participated in two recent advanced trials was likely diminished by exposure to the virus through anal sex. Because HIV transmits much more readily through anal versus vaginal intercourse, even if a small proportion of women’s sex acts are anal, those acts could nevertheless cause a considerable drag on the efficacy of a product that provides ARV coverage only in the vagina. This particular conundrum drives Dieffenbach’s preference for long-acting systemic products that cover both orifices, such as long-acting injectable PrEP or, perhaps one day, an implant that provides sustained release of prevention medication.

When the University of Washington’s Jared Baeten, MD, PhD, announced results from a key trial of the dapivirine vaginal ring at the 2016 Conference on Retroviruses and Opportunistic Infections in Boston, he was met with a standing ovation.Courtesy of Benjamin Ryan
Dieffenbach also doubts that the for-profit pharmaceutical sector or the small handful of large nongovernmental organizations that can afford to manufacture a vaginal or rectal microbicide will anticipate a large enough market for a vaginal or rectal microbicide to warrant undertaking large-scale production to make such a product widely available.
“There will be winners and losers, particularly when we get past early clinical trials,” Dieffenbach says of the future of HIV prevention research. “Decisions have to be made about what we will spend our money on. And as products improve, the barrier goes higher and higher.”
Currently, eight earlier-stage NIH-funded safety studies of rectal microbicides studies are under way, including those looking at medicated inserts, suppositories, gels, enemas and lubricants. And the MTN may soon launch two more such trials. Additionally, more than 10 vaginal microbicides are in development, including multipurpose modalities that, because they intend to prevent pregnancy or other sexually transmitted infections (STIs) in addition to HIV, will likely prove more appealing to women than those microbicides that serve only one function.
Dieffenbach does see promise in next-generation vaginal rings that would perhaps provide three months of HIV prevention or also include contraception. As for the rest of the microbicide pipeline, he says he prefers that researchers go back to the drawing board and devise much more revolutionary HIV prevention products—perhaps a form of post-exposure prophylaxis (PEP) to rival the simplicity of the Plan B contraception method, requiring only one or two pills that can provide sustained-release medication following potential exposure to the virus.
Pointing to the ever-unpopular female, or “internal,” condom—“a good example of a product still looking for a home”—Dieffenbach says he is particularly concerned that the desire for and adherence to microbicides among key at-risk populations will prove limited, a perception advocates of microbicides adamantly dispute.
Dieffenbach does, however, support continuing the current microbicides trials. And should any of them yield promising results in the coming years, he says the DAIDS funding apparatus will maintain the flexibility to move such microbicides into advanced research. But given Dieffenbach’s statements that research beginning in the next decade should probably not focus on topical microbicides, advocates are worried that the DAIDS director has effectively shut the door on the continuation of research ventures that won’t produce results until after the launch of the next funding cycle.
Microbicides: A Victim of PrEP’s Success?
PrEP has set a high bar for the HIV prevention field, apparently proving close to 100 percent effective among individuals who can access as well as tolerate Truvada (in general, the drug is quite well tolerated) and who adhere to the daily regimen. Even on a population level, PrEP reduced HIV by an impressive 86 percent in one real-world British study of very high-risk men who have sex with men (MSM). Among patients receiving PrEP from Kaiser Permanente Northern California, none who have stuck with the regimen have contracted the virus during a cumulative 5,000 years of Truvada use.

A controversial billboard promoting PrEP in West HollywoodCourtesy of the Los Angeles LGBT Center
Meanwhile, the NIH has spearheaded the launch of six major advanced trials of HIV prevention products, with results expected in the early 2020s, that generally meet Dieffenbach’s long-acting and systemic requirements. Two pairs of trials are looking at two forms of long-acting injectable prevention given every eight weeks: One pair of studies is investigating the broadly neutralizing HIV antibody VRC01 as prevention; another pair of trials is testing a long-acting formulation of the ARV cabotegravir as PrEP. And two different vaccine trials are now under way in sub-Saharan Africa.
Dieffenbach’s skepticism about microbicides is driven in no small part by their laggard status. They must compete for support with a healthy handful of heavy-hitting products that are much further along in the pipeline. Microbicides researchers, meanwhile, argue that there is enough room in the pipeline for nonsystemic modalities, which they argue are desperately needed as a means of offering an array of prevention products to better fit variable personal needs.
“The current strategy of saying there would be a single prevention network and all prevention has to be systemic would quite simply put us out of business,” says Sharon Hillier, PhD, a professor of obstetrics and gynecology at the University of Pittsburgh and a co–principal investigator at MTN.

Sharon L. Hillier of the University of Pittsburgh at the 2017 Conference on Retroviruses and Opportunistic Infections in SeattleBen Ryan
Myron Cohen, MD, chief of infectious diseases at the University of North Carolina at Chapel Hill, who led the landmark HPTN 052 study that in 2011 first proved that standard ARV treatment is associated with a marked reduction in HIV transmission risk, is more sanguine in his appraisal of Dieffenbach’s vision.
“I don’t think they’re trying to wipe out a whole field,” Cohen says of NIH’s intentions. “I think they’re trying to readjust their portfolio toward investments that they think will have a greater likelihood of success in a moment in time.”
Craig W. Hendrix, MD, a clinical pharmacologist who studies microbicides at Johns Hopkins University School of Medicine, acknowledges that DAIDS faces a difficult decision about how to best spend research funds that, even in their immensity, remain limited. “But it seems like all the eggs are in the long-acting cabotegravir basket,” Hendrix says. “I think the basket is friable. That to me is a bit risky.”
Hendrix advocates parallel advanced studies of rectal and vaginal microbicides to provide new prevention modalities for those who simply do not want to take a systemic medication for the long term and also to hedge against the possible failure of the cabotegravir PrEP trial.
Dieffenbach, on the other hand, says the data he’s reviewed give him doubts that rectal microbicides will provide adequate enough ARV coverage in relevant tissues to confer a high level of protection against HIV acquisition through anal sex.
In an effort to bring to market appealing HIV prevention products, Hendrix and others in his field have designed microbicides that piggyback on men’s existing sexual behaviors. For example, many MSM already use anorectal douches to clear the runway, so to speak, for receptive anal intercourse. So providing these men with a medicated version of a product they already use would at least in theory buttress their adherence to a microbicide-infused enema.
Advocates for microbicides are further concerned that the NIH will foreclose upon the development of products that, by virtue of being nonsystemic, have much lower risk of producing significant side effects and are easily and quickly reversible. Women may look much more favorably on, say, a dissolvable film placed in the vagina that dissipates within days or weeks compared with a long-acting injection of cabotegravir that may linger in the body for as long as a year.
Microbicides, especially those that can be distributed cheaply by workers who don’t require significant medical training, may also help bring down HIV infection rates without overburdening health care workers who are already stretched thin, especially in the developing world. What’s more, a prevention product at-risk sub-Saharan Africans could obtain from an outreach worker who visits their village from time to time could be an appealing alternative to attending medical appointments every eight weeks for a long-acting injection.
“If you really want something that’s simple and cheap to implement and doesn’t burden the health system,” stresses Hillier, “you take things that are not systemic.”
Cohen, a co–principal investigator of the HPTN network, believes that an NIH and DAIDS shift away from microbicides development would be practical because of the way that advanced studies of new HIV prevention modalities will likely need to be structured going forward. The architects of such trials face a thorny challenge: They must strike an ethical balance in which participants are not denied the opportunity to receive a highly effective existing product—right now that means Truvada as PrEP, but in the coming years it could mean a long-acting injection—just to test the efficacy of a new product, such as a vaginal or rectal microbicide that initial research may have indicated may be only moderately effective.
Of course, many people who engage in condomless intercourse cannot take PrEP—whether because of side effects or lack of access—or they simply will not take any systemic medications. So when provided with the opportunity to use a microbicide that reduces their risk by, say, 70 percent, their personal alternative is not the 99 percent effectiveness of PrEP but rather no protection at all. According to microbicides research advocates, this very real-life equation could support the ethical design of even moderately effective microbicide products.
“Most important,” Cohen says, participants in such a trial would “need to know with some precision the protection they are getting so they can make well-informed choices.”

Myron Cohen, MD, speaks at the 9th International AIDS Society Conference on HIV Science in Paris in July 2017Benjamin Ryan
Decision Time
Jim Pickett, the fiery and outspoken senior director of prevention advocacy and gay men’s health at AIDS Foundation of Chicago, lambastes Dieffenbach for “magical thinking” in his skepticism about microbicides. In his capacity as chair of IRMA (International Rectal Microbicides Advocates), Pickett has circulated two letters urging Dieffenbach and Anthony S. Fauci, MD, the director of NIAID, to maintain robust support for topical microbicide development. More than 150 organizations and 350 individuals have signed IRMA’s rectal microbicides–focused letter, while 93 organizations and 175 individuals have added their names to a female-centric letter.
Additionally, Pickett’s efforts have led 18 members of Congress to sign a letter written by Representative Jan Schakowsky, a Democrat from Illinois, calling upon the same from Dieffenbach and Fauci.
“I’m committed to having folks have choices that work for them,” Pickett says of his hopes for a future with multiple effective HIV prevention products in the proverbial toolbox. He claims that Dieffenbach and his colleagues seek “to drastically limit choices for men and women around the world.”

Jim Pickett, a long-time PrEP advocate, gives an address on the subject at the 2016 Conference on Retroviruses and Opportunistic Infections in Boston.Courtesy of Benjamin Ryan
Cohen is keen to stress that he does not see the debate about DAIDS’s future research priorities as an “us versus them” situation, with HIV advocates squarely pitted against the NIH bureaucracy.
“The NIH wants to support research that maximizes prevention of HIV,” Cohen says.
In a culmination of all this debate, the NIAID’s AIDS Research Advisory Committee, made up of 13 voting members who do not work for the federal government, the lion’s share of whom are leading HIV research scientists, will meet in January to hear Dieffenbach’s plans for the 2020 to 2027 DAIDS funding cycle.
The committee, which includes Hillier, has been working with Dieffenbach and his NIH colleagues throughout 2017 to provide feedback and raise concerns about the agency’s designs on the future. If they vote to reject Dieffenbach’s proposed research priorities—he does not have a vote—the DAIDS head will be in a position to respond to their suggested changes.
However, at the end of the day, the committee can only advise. Leaders at the NIH, including Fauci, have the final say-so; although Dieffenbach, who heads this decision-making effort, emphasizes that the January meeting is no mere formality.
As for how amenable to public comment he has been thus far, Dieffenbach says, “I listen. At the same time, I have a belief system,” one that he says is guided much more by data than sentiment.
Benjamin Ryan is POZ’s editor at large, responsible for HIV science reporting. His work has also appeared in The New York Times, New York, The Nation, The Atlantic and The Marshall Project. Follow him on Facebook, Twitter and on his website, benryan.net.






5 Comments
5 Comments